Aktualności
Impact Stars Award – Deloitte Technology Fast 50 Central Europe
Neuro Device Group S.A. is among the fastest-growing technology companies in Central Europe! This is a huge honor, which – immodestly speaking – we worked hard for. This edition of the Deloitte Technology Fast 50 Central Europe was record-breaking – out of over 450 submissions, more than 140 were from Poland, including ours. We would […]
Read more
Neuro Device Group S.A. is relocating!
We are moving from Praga-Południe to the beautiful, green Żoliborz district. Our email address and phone numbers remain the same, but from now on you can find us at ul. Gen. Zajączka 28, 01-510 Warsaw.
Read more
NeuroTech Talk
Krzysztof Malej, Head of R&I took part in the NeuroTech Talk event. Krzysztof was talking about Voic – our comprehensive system for post-stroke aphasia therapy.
Read more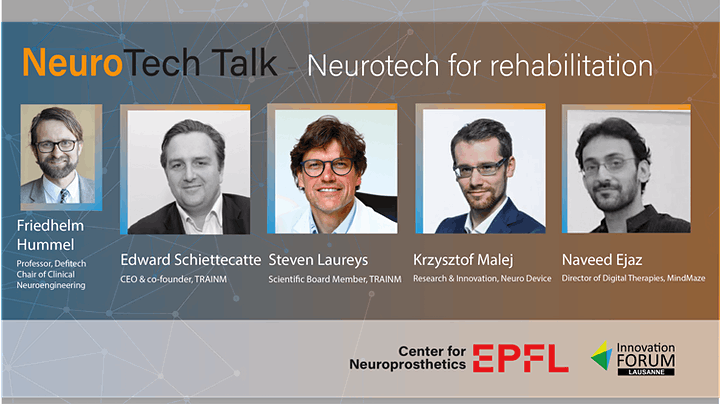
Supermeni Jakości plebiscite by TUV NORD Polska
We have been nominated in the TUV NORD Polska Supermeni Jakości plebiscite in the SECURITY (BEZPIECZEŃSTWO) category. The voting continues until the end of September. Each person can vote once by providing their email address to which a link to the candidates’ list will be sent (from which you should pick us:). If the mission […]
Read more
International Conference Planet Masters
On August 21 Paweł Soluch had the pleasure of speaking at International Conference Planet Masters with the Grow Your Company program. Paweł talked about his leadership, how he managed to create a team and lead it a Freedom Focused Company. He also talked about the Voic project, which is on its best way to improve […]
Read more
Neuro Device is having 40 trees planted!
Environmental awareness is deeply rooted in the Neuro Device culture. That is why we’ve decided to celebrate the 40th birthday of our CEO by having 40 trees planted through Posadzimy.pl. The trees will be planted this autumn in the Ogrodzieniec community, where a natural disaster caused a severe loss to the forest stand. Our seedlings […]
Read more
nurostym tESm certified as a medical device!
We are pleased to inform that at the end of May 2021, we had our electric brain stimulator nurostym tESm certified as a medical device, in accordance with the new European regulation on medical devices. The certification includes tACS and tRNS current modes what makes nurostym tESm the first medical device of its kind in […]
Read more
The conclusion of the LifeTone domestic tests
With the end of March, we have concluded domestic tests of the LifeTone Baby Monitor. Several dozen children under 12 months participated in the study. We have managed to prove, that our breathing and life functions monitor works perfectly for children within this age group. Many thanks to all the parents of the children, who […]
Read more
AfatES – participants’ testimonies
Our study aimed at people suffering from poststroke aphasia is underway. The volunteers for the study have many questions regarding the inclusion criteria, as well as the study itself. Neurostimulation still stirs a lot of emotions, so we have decided to give the public a closer look at the project. We invited three of its […]
Read more
The CAMRI System in help of the youngest.
Our patients communication system – CAMRI, is designed to work in the MRI environment. It was installed in a specialist neonatal incubator, bought with the money raised during the 27th final of The Great Orchestra of Christmas Charity (WOŚP). The photos were sent to us by doctor Przemysław Podgórski from the University Clinical Hospital of […]
Read more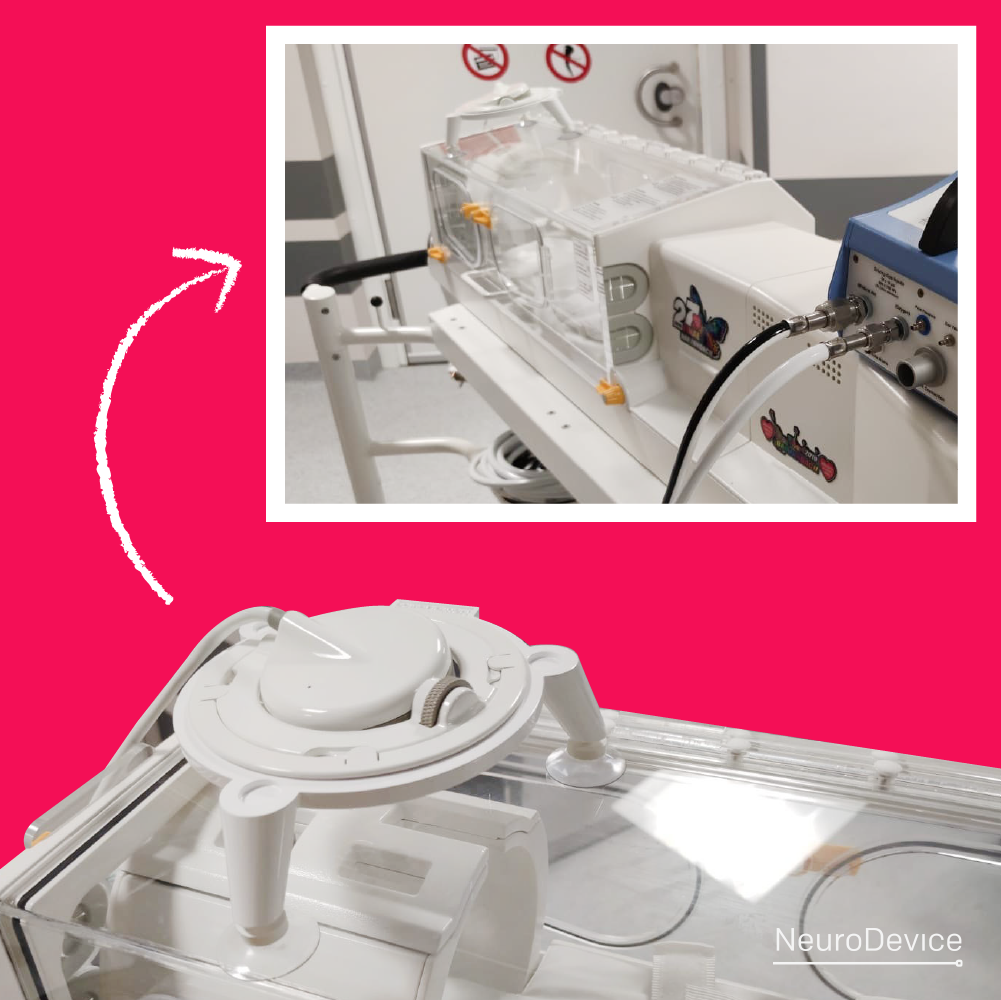
What will 2021 in our industry be like?
After a pandemic year, it basically seems obvious that 2021 should only get better. But that is too general a statement to agree with. Moreover, Covid-19 still wins against us, not us against it, so any scenario is possible. I also believe that this year will continue to be marked by great uncertainty. Looking beyond […]
Read more